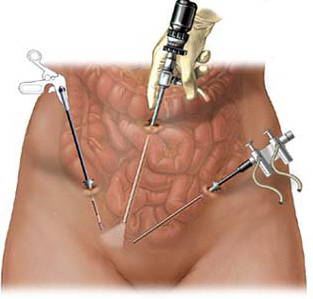
The power morcellator, a medical device that found FDA approval in 1995, is used to remove the uterus in approximately 50,000 – 75,000 hysterectomies each year. However, there are studies that show that morcellation has been linked to an increased risk of spreading cancerous cells around the abdomen region. Have you undergone a hysterectomy with the use of a power morcellator? Were you subsequently diagnosed with cancer in your abdomen? You may be entitled to compensation. The morcellation attorneys with Arentz Law Group P.C. believe that the manufacturers of this device knew the risks, but failed to warn the public about those risks. Contact an attorney today by calling 1-800-305-6000 or by filling out the contact form on this page to schedule your free initial consultation.
What is a Morcellator?
A power morcellator is a special tool that consists of a tube with a set of cutting blades on the end. This device will simultaneously cut off, and shred the portion of the uterus that is known to be cancerous. The pieces of cancerous fibroids, or the entire uterus, can now be removed via the small incision the doctor has made.
What is a Hysterectomy?
As anyone ages their body tends to wear out. Joints become worn, eyes no longer see properly, hearts become weak, and overall the body simply ages. The uterus is no exception to the rule. This muscle, that is essential for childbirth, is also prone to various problems. Cancer can develop and fibroids can form, it can shift out of place, heavy bleeding and severe cramping can ensue. For over half a million women each year the answer is to simply have it removed. By having a hysterectomy, or removal of the uterus, women hope to relieve the discomfort, and prevent uterine cancer from forming.
Out of the hundreds of thousands of hysterectomies performed each year, about 20% are done laparoscopically. This procedure is minimally invasive, and allows the patient to experience a much more rapid recovery than if traditional open surgery were performed. In fact, the procedure can be done through the belly button or through the vagina to ensure that no visible scars are left after healing.
In order to perform a laparoscopic procedure, specialized tools must be used. The surgeon requires a light and a camera in order to see what he or she is doing, and they need a device to cut and remove the tissue. The device that has become popular over the years is a morcellator.
The Problem with Morcellators
Morcellators are manufactured by many different companies, but the most popular is the Gynecare Morcellator made by Johnson & Johnson. This device is essentially a long tube with cutting blades on the end. The blades cut away the tissue to be removed, grinding it up so it can be extracted through the tube. This method makes quick work of the procedure, and allows the patient to recover 4 times as quickly than if she were to have undergone a traditional hysterectomy.
The problem, however, is that the morcellator does not remove all of the tissue. Some pieces, or fragments, are left inside the patient, and one in 350 women have unsuspected sarcoma (a form of cancer) when they opt for this surgical procedure.
The fibroids on the uterus may be latent sarcomas. That is, they may be cancerous, but in a dormant stage. By spreading these cells around the area, the cancer begins to grow rapidly and aggressively. A patient who went in to have a morcellation hysterectomy to prevent cancer and relieve discomfort is now experiencing a life threatening cancer battle.
History of Morcellation Devices
Most hysterectomies (removal of the uterus) and myomectomies (removal of fibroids) are done through a traditional surgery. The surgeon makes an incision on the abdomen, and performs the work that way. However, many women are seeking out less invasive procedures. These minimally invasive procedures make just a small incision, and then with specialized equipment the surgeon can do the work without fully opening the area. The result is that the patient experiences less pain, and a faster recovery.
Since 1995, one tool used for some of the minimally invasive surgeries is the morcellation device. Nearly 20 years ago this device found FDA approval, and since then nearly two dozen similar devices have hit the market. Consisting mainly of a tube and a cutting blade, the device is inserted into the uterus. It then cuts out the offending tissue, chopping it into small pieces, and sucking it back through the tube in order to remove the uterus or fibroids.
For the next 17 years this method of tissue removal gained popularity. Many patients were finding the procedure to be effective, and the recovery time to be lower. However, research that was published in 2012 showed some shocking evidence that the use of a morcellator could in fact cause the spread of leiomyosarcomas, or cancer of the soft tissue.
The morcellator would chop the offending tissue into pieces, most of which would be sucked out of the body. However, some of the cancerous material would be flung around inside the uterus or, in the case of uterine removal, flung around in the pelvic region. These malignant pieces of tissue would cause the spread of cancer much more aggressively. In fact, the rate of unexpected sarcoma after the procedure was found to be 9 times higher than previously stated.
Morcellation Devices
In 1995 the FDA approved the first morcellator. Over the next 19 years nearly two dozen of these devices have been approved as medical devices through the FDA’s 501(k) program. An estimated 50,000 – 75,000 women have their hysterectomies or myomectomies performed with the help of a morcellator. Some of the most commonly used are:
- Diva Morcellator manufactured by FemRx (owned by Johnson & Johnson since 1998)
- MyoSure manufactured by Hologic
- VersaCut Morcellator manufactured by Lumenis Inc.
- Gynecare X-Tract manufactured by Ethicon (A Johnson & Johnson company)
- Gynecare Morcellex Tissue Morcellator manufactured by Ethicon
- Morcellex Sigma manufactured by Ethicon
- Hysteroscopic Morcellator manufactured by Interlace Medical (acquired by Hologic in 2011)
- Trueclear Hysteroscopic Morcellator manufactured by Smith & Nephew
- PlasmaSORD manufactured by Olympus
There are numerous other devices on the market, yet they all perform the same function.
Gynecare Morcellator
In 1998 a subsidiary of Johnson & Johnson, the world’s largest medical device manufacturer, called Ethicon started manufacturing these devices. They came out with three different models that have been on the market for many years. The Gynecare Morcellex, Gynecare X-Tract, and Morcellex Sigma are the most widely used power morcellators. They account for a significant portion of the procedures that are performed on women.
In 2012 a study was published on the PLOS ONE website. It showed that these devices, while they did a great job at minimizing damage to surrounding tissue, they were actually causing the spread of cancerous cells around the abdomen. In fact, the dangers of having cancer spread were 9 times greater than previously stated.
Johnson & Johnson responded to the warning from the FDA by pulling their Gynecare Morcellator, and their Morcellex Sigma from the shelves. They did not, however, issue a recall for any devices that were already in hospitals across the country. They stated that they will keep the devices off the market until “their role is better understood and redefined by the medical community.”
Using a Gynecare morcellator is easier for the surgeon, allows the patient to recover in just 2 weeks instead of 8, and has many other benefits. Unfortunately the risks of spreading cancerous cells, those which may not even be known until it is too late, is far greater than the manufacturing companies ever acknowledged.
Lumenis Morcellators
In 1992 Lumenis Incorporated opened its doors. Located in Salt Lake City this medical device company makes products for the aesthetic, surgical, and ophthalmological communities. They have over 200 patents and have been a leading developer of medical devices for many years.
While the company is prevalent in the medical community, it is not a giant corporation like many of its competitors. Holding a market cap of almost $550 million puts it at the lower end of the range. But that is not to say they are small. In the first quarter of 2014 the company posted earnings of over $65 million, an increase of almost 10% from the year before.
Many of these earnings came from the sales of their power morcellation device, the VersaCut. This device acts just like the other devices and is used to remove fibroids in the uterus that are bothering the patient. Since its initial approval in 2005, the device has been used to perform surgery on thousands of women in hopes of shortening recovery times.
Unfortunately, what happens in a good portion of women who have their surgery performed with a VersaCut morcellator is that cancer spreads throughout the uterus or abdomen region. When the morcellator chops and grinds the tissue, some of it is spread through the area. If this tissue contained cancerous cells, then that cancer is “seeded” around the area. The seeded cancer then starts to grow aggressively, and the patient can go from no cancer symptoms, to an advanced stage of cancer in just a matter of days.
Hologic Morcellators
Hologic, a company dedicated to designing and manufacturing medical devices for women’s health, was founded nearly 30 years ago in 1986. Since then they have grown and expanded to become one of the leading device manufacturers in the country. The company sees massive sales, with revenue for 2013 at nearly $2.5 billion. Their device MyoSure is one of the top selling morcellators on the market today and has greatly helped to bolster sales in this company that has a market cap of almost $6 billion.
The MyoSure morcellation device was approved for use in 2009. This thin tube is inserted through the dilated cervix, where it can then be used to remove fibroids and polyps. Because the device is so small, and it is not used for total uterus removal, there is no surgery required, not even a small incision. Due to this fact, the manufacturers can brag a very rapid recovery rate.
While this device may be better at removing the tissue, and even though the MyoSure website boasts that nearly 100% of the tissue is removed every time, there is always some that is left behind. When a power morcellator is used, the tissue is ground up and removed from the body. When this happens some is left and some is scattered through the uterus. If there are cancerous cells in the tissue that is left behind or scattered, it can spread the cancer to other areas of the uterus. When spread, the cancer begins to aggressively grow and a patient can go from no cancer symptoms, to an advanced stage in a short period of time.
Smith & Nephew
Nearly 150 years ago London, England saw the founding of the medical equipment manufacturer Smith & Nephew. This medical giant has since expanded to countries around the world, and accumulated a massive stockpile of assets. Their current market cap is over $15 billion and the first quarter of 2014 saw revenues coming in that were more than $1 billion. These earnings were helped by their product, the TruClear Hysteroscopic Morcellator.
The TruClear Morcellator system initially found FDA approval in 2011. With a tube diameter of just 5mm, the device can easily be inserted through the cervix to access the uterus; it is currently the smallest morcellator available. While the manufacturer boasts that there is no tissue floating in the uterine cavity due to continues outflow and suction, it has been found that all morcellators leave behind some particles.
These tissue particles may end up causing no harm to the patient. However, it has been found that 1 in 350 women have unsuspected leiomyosarcoma. That is, they have cancerous fibroids that have yet to be diagnosed as cancerous. If these fibroids are chopped and removed, the cancerous cells can be seeded throughout the area. This seeding causes the cancer to spread and grow much faster than if it were left alone. The result is that the patient finds them very quickly suffering from an advanced stage of cancer, and they are faced with the prognosis that their long-term survival outlook is bleak.
FDA Morcellation Response
FDA Approval
The
first power morcellator found FDA approval nearly 20 years ago. In the spring of 1995, this simple tool was granted approval through the FDA’s 510(k) process (that is, the process for approving medical devices). Since that time many more devices have found FDA approval. There are currently 15 top selling devices out there that are still in use in hospitals around the country.
FDA Retraction
The device has not been “disapproved” for use; however recent research has led the FDA to announce that they now discourage the use of these devices. In April, 2014 the FDA concluded that the devices were not entirely safe for the use of hysterectomies or myomectomies (removal of uterine fibroids).
This statement came following a shocking discovery. Research that was published on PLOS ONE in late 2012 showed that women were put at much more risk when a morcellator was used during their surgery than if a traditional open belly operation was performed. This research showed that the rate of unexpected sarcoma (a type of cancer) was actually 9 times more likely than previously stated.
Johnson & Johnson Response
Johnson & Johnson’s subsidiary Ethicon has manufactured and sold 3 different morcellators. On the heels of this announcement from the FDA J&J has announced that they will cease the sales of their devices.
The company, however, does not indicate any plans for a recall of the devices already in use; a troubling idea for morcellation critics. The giant corporation does not plan to sell anymore, but they are leaving the devices out there to be used over and again despite the high rate of spreading cancer.
Morcellation Lawsuits
There have already been a number of suits filed against the companies that manufacture these devices. The belief is that the manufacturers knew of the dangers, yet failed to warn their clients that the use of such device could increase the risk of the cancer spreading. Just a few examples are below.
Brenda Leuzzi
In late 2012
Brenda was having trouble with fibroids hemorrhaging. Her doctor performed a routine myomectomy, and in the process removed a tumor that was in the low stages of cancer growth. However, during the morcellation process the cancer cells spread, and has caused Brenda’s cancer to leap to an advanced stage of development. Her suit against
Ethicon (a branch of Johnson & Johnson) is ongoing.
Dr. Amy Reed
Dr. Reed went in for a routine hysterectomy via morcellation. As a busy professional with a large family, Dr. Reed was relying on the shortened recovery time with a minimally invasive surgery. However, after her surgery she was informed that she had uterine leiomyosarcomas, and the morcellation process may have spread the cancer around her abdomen.
Amy Reed was an anesthesiologist at a prominent hospital in Boston. At 40 years old she was just taking off in her career, making a name for herself by treating the Boston Marathon bomber. In October 2013 Amy had given birth to 6 children, and her uterus was acting up. Undergoing what she thought would be a routine surgery has forced her to battle for her life.
During and after childbirth uterine fibroids can act up. These mostly benign tumors are usually just uncomfortable and rarely life threatening. This spurs many thousands of women to undergo a non-medically essential hysterectomy. During Amy’s laparoscopic hysterectomy it was discovered that she had leiomyosarcoma, a malignant cancerous fibroid.
Amy’s procedure was performed with the use of a power morcellator. This device grinds up the tissue and extracts it through a small tube in order to keep the surgery as minimally invasive as possible. However, when grinding a cancerous tumor the cells can be spread through the abdomen region. This causes the cancer to grow much more rapidly and spread much more easily than if the tumor were removed intact.
Amy is now facing up to an 80% chance that her cancer has spread; as opposed to a 30% chance of it spreading had a morcellator not been used. She is currently undergoing chemotherapy, and has stage 4 cancer. Her prognosis is that she will most likely pass away within 5 years. She and her husband are petitioning to have morcellation removed as an option until a safer method can be maintained.
You can read more about Amy Reed and her battle on the Wall Street Journal Website.
Scott Burkhart
Scott has filed a negligence and wrongful-death suit against Ethicon, Blue Endo, and LiNA Medical after his wife Donna passed away from metastatic leiomysarcoma. Donna went in for a routine hysterectomy in March 2012 with no signs of cancer. However, just nine days later she was diagnosed with the cancer that ultimately took her life in February 2013, not even one year after the surgery.
Contact Arentz Law
The highly trained morcellation attorneys with Arentz Law believe that the manufacturers of these devices knew that the risks were as high as they are, yet they failed to warn the public. Subsequently hundreds more women have been subjected to cancer than was necessary. If you, or someone you love, have undergone a hysterectomy procedure where a power morcellator was used, and you were subsequently diagnosed with widespread cancer, you may be entitled to compensation. Contact a morcellator lawyer from Arentz Law right away by calling 1-800-305-6000 or by filling out the contact form on this page to schedule your free initial case review.
Attorneys with Arentz Law can represent clients who live in all 50 states.