St. Jude Durata Lead
Experienced St. Jude Durata lead lawyers at Arentz Law Group, P.C. are closely monitoring the investigation into the safety of this potentially defective product after the recall of its predecessor, the St. Jude Riata lead, in 2011. The St. Jude Durata lead connects a defibrillator to the heart, and the FDA has suggested that quality control problems at the St. Jude Medical plant in Sylmar, California may have led to defects that could have life-threatening consequences. If you or someone you love has suffered injury or wrongful death that you believe may be linked to a faulty St. Jude defibrillator lead, you may be able to pursue substantial compensation for your damages in a dangerous product liability lawsuit. Become fully informed of your rights: contact us to schedule a free consultation with a St. Jude Durata attorney.
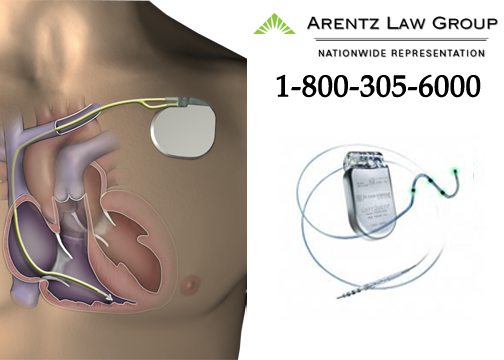
What is the St. Jude Durata Lead?
St. Jude Medical, a medical device company based in Minnesota, makes the Durata lead. The St. Jude Durata lead is a tiny wire that connects a defibrillator to the heart. A defibrillator, located outside the heart, monitors heart rhythm and delivers a high-voltage shock to the heart if serious rhythm abnormalities are detected. Connected directly to the heart tissue, the Durata lead serves to carry information between the heart and the defibrillator. If necessary, the lead also delivers the high-voltage shock when necessary.
St. Jude Durata Lead Problems
The St. Jude Durata lead is almost identical to the St. Jude Riata lead, which was recalled in November 2011 after numerous reports of premature failure due to premature corrosion of the insulation that protects the leads. If a defibrillator lead corrodes, it can cause the conducting wires it houses to move deeper into the lead or externalize outside the lead. This can lead to a number of problems that could culminate in cardiac arrest:
- Improper pacing or sensing of the heart rhythm
- Inappropriate delivery of shocks to the heart
- Failure to deliver life-saving shocks to the heart
Though the Durata lead differs from the Riata lead in that it is coated in what is meant to be a safer and more durable material, there is growing concern that similar design defects plague both devices. In fact, to ensure that the Durata lead does not share the Riata lead’s defects the FDA conducted an in-depth inspection of St. Jude Medical’s manufacturing facility in Sylmar, California in late 2012. 11 problems were identified with the manufacturing and quality control protocols at this facility. Based on these findings, the FDA publicly warned St. Jude Medical that a failure to address the problems identified would result in “seizure, injunction, or civil monetary penalties”. The agency also made it clear that they would not approve new, similar medical devices made at this facility, nor would they allow export requests from it until St. Jude Medical fixed the problems at that site.
Contact a St. Jude Durata Lead Lawyer
To date, no St. Jude Durata lead recall has been issued, though the device continues to be investigated. The FDA has received at least one adverse event report concerning the Durata lead, indicating that a Durata lead had to be removed because the wires were poking through the insulation. The St. Jude Durata lawyers at Arentz Law Group, P.C. are closely monitoring all studies focused on St. Jude leads, which we fear may exhibit similar problems to the recalled Riata. If you or someone you love suffered injury or worse due to a faulty St. Jude Durata or Riata lead, you could be entitled to compensation. We encourage you to contact a St. Jude Durata lawyer to schedule a free legal consultation as soon as possible.
